
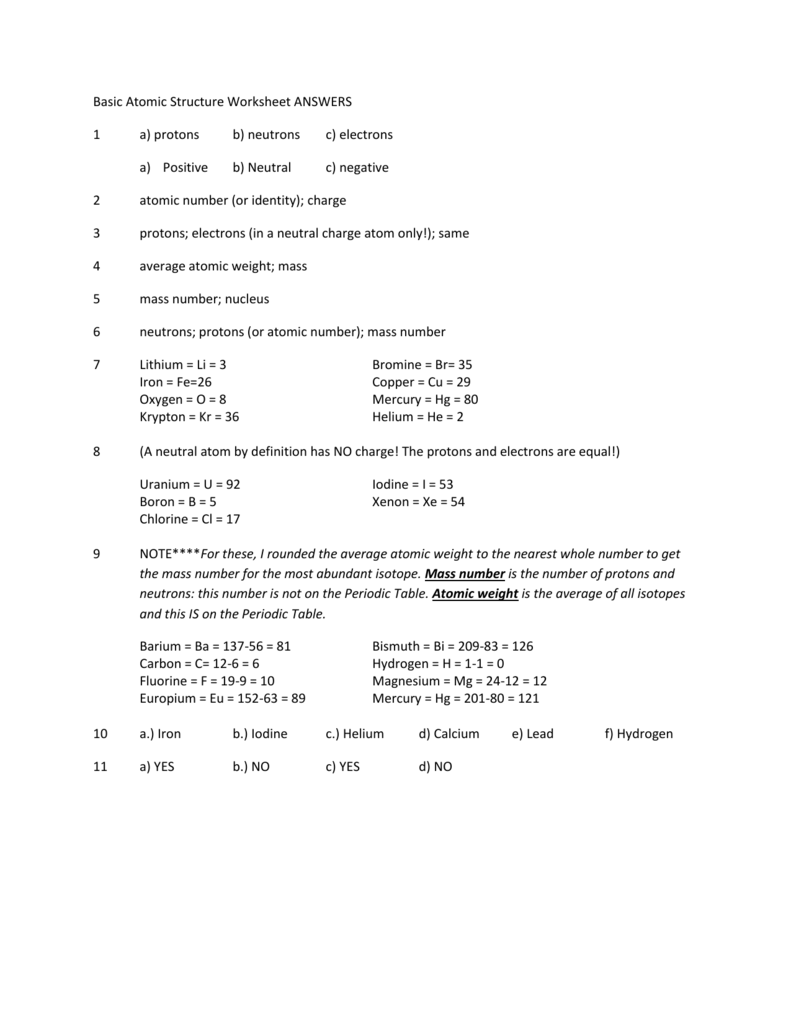
You can estimate the relative amount of your peptide/protein, the purity of the sample, or even the fold of the. Background The Rydberg Formula for the wavelength of the radiation emitted in atomic energy level transitions is 1 ZR1 - 1) Infinal ninitial where R 1.097x10'm-1, Z is the atomic number number of protons) of the element, Nfinal is the principal quantum number. of celestial spectra is only well begun, and that lab-. Lab 9 Atomic Line Spectra and PHYS 112 Atomic Structure Name: I. Determination of term type from electron configuration.
Still in print in 2011, Atomic Spectra and Atomic Structure is now in its 36th Dover printing, by far the record for any Dover scientific book. In this model n = ∞ corresponds to the level where the energy holding the electron and the nucleus together is zero. PRE-LAB QUESTIONS When an element is subjected to energy (electricity or heat), its electrons begin to jump back and forth between energy levels. Question: EXPERIMENT REPORT SHEET Atomic Spectra and Atomic Structure 12 Calibration of Spectroscope Lines observed in emission spectrum of mercury Color Position on scale Known wavelength violet 1.71 404.7 nm bile 3.62 432.8 nm queen 7.46 546. Answer In general, NMR is very informative and powerful technique. theory to describe the properties of atoms as we know them today. Building-up Principle and Periodic System of the Elements: 1. His classic work on spectroscopy, Atomic Spectra and Atomic Structure was published by Prentice-Hall in 1937 and first reprinted by Dover, with corrections, in 1944. This quiz and worksheet allow students to test the following skills: Reading comprehension - ensure that you draw the most important information from the related lesson on atoms. Read the lab thoroughly and answer the pre-lab questions that appear at the.
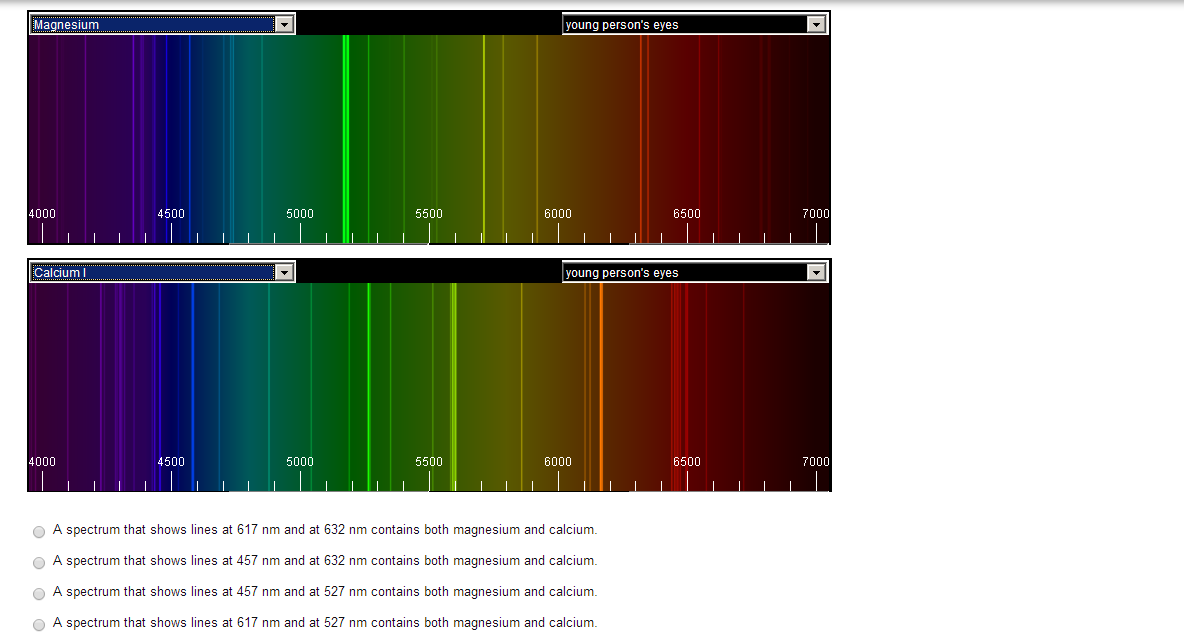
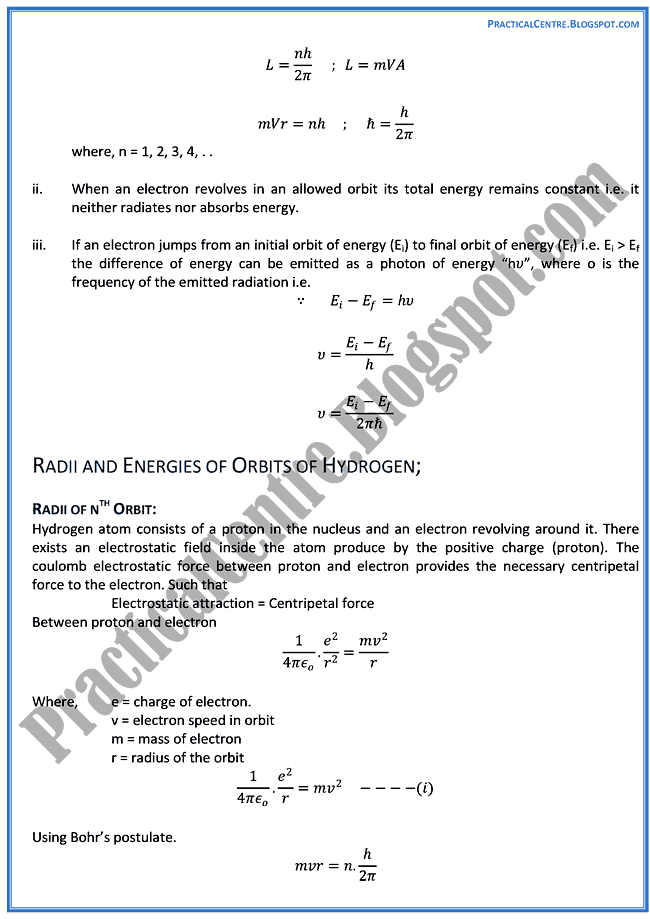
When exploding fireworks, you will see the color when the metal atoms absorb energy from the detonater.\) is the Rydberg constant in terms of energy, Z is the atom is the atomic number, and n is a positive integer corresponding to the number assigned to the orbit, with n = 1 corresponding to the orbit closest to the nucleus. Atomic Spectrum of Hydrogen Prelab Assignment Before coming to lab: Use.(CC BY-NC-SA 3.0 Christopher Auyeung via CK-12 Foundation) Question: On the top of the spectrometer, it indicates that fluorescent lights should produce a spectral line at around 577 and 579 nm, in addition to the wavelengths shown in the first column of the table above. The average distance between the electron and the nucleus isproportional ton2,inaccordance with Bohr’s model of the hydrogen atom, which predicts that the classicalradius of the electron orbit should grow withnasa0n2,a0 being the Bohr radius(a00.52917721092(17)×1010m).


 0 kommentar(er)
0 kommentar(er)
